

Home » Services » Clinical Data Management
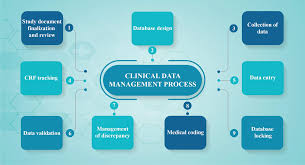
Cinical Data Management Clinical Data Management (CDM) ensures data integrity throughout the research, making sure datasets are accurate, secure, reliable, and ready for analysis. By using a systematic process, a CDM team are responsible for collecting, entering, cleaning and processing information gathered during a clinical research project, and ensure the accuracy, completeness and consistency of the clinical trial data, adhering to regulatory standards and guidelines. The main goal of CDM is to ensure the validity and reliability of the data, so it's ready for regulatory submission and subsequent analysis. It serves as the backbone for producing high-quality, statistically sound results that inform medical decisions and regulatory approvals. Regulatory compliance is a fundamental aspect of CDM, ensuring that all collected data adheres to stringent regulatory standards and guidelines set by authorities such as the FDA and EMA. By maintaining records and implementing robust validation processes, CDM guarantees that the data adheres to regulatory requirements to not only facilitate the approval process for new treatments and interventions but to also uphold the integrity of the research. The emphasis on data quality is paramount, as it directly impacts the credibility of research outcomes. High-quality data ensures that the results of clinical trials are statistically sound and dependable, forming a solid foundation for medical decisions and ultimately advancing patient care. In summary the purpose of Clinical Data Management is to: --Ensuring Data Quality and Integrity --Compliance with Regulatory Standards --Data Collection and Management --Facilitating Accurate analysis and Reporting